ARTIGO
Fisiopatologia da DPOC e cascata inflamatória
Você sabia que diferentes tipos de inflamação crônica têm um papel importante na fisiopatologia da DPOC? Saiba mais sobre a fisiopatologia da DPOC e cascata inflamatória nesse artigo.
DPOC: uma doença pulmonar crônica e inflamatória
A DPOC é uma doença pulmonar heterogênea, prevenível e tratável, caracterizada por sintomas respiratórios persistentes, limitação do fluxo de ar e suscetível a possíveis exacerbações.1
DPOC - Definição GOLD
“A Doença Pulmonar Obstrutiva Crônica (DPOC) é uma condição pulmonar heterogênea caracterizada por sintomas respiratórios crônicos (dispneia, tosse crônica, e aumento da produção de muco e/ou escarro) devido a anormalidades das vias aéreas (bronquite, bronquiolite) e/ou acometimento dos alvéolos (enfisema pulmonar), que causam obstrução persistente, muitas vezes progressiva, ao fluxo aéreo”1
Certos fatores de risco podem desencadear a inflamação crônica das vias aéreas que leva ao desenvolvimento da DPOC1,4,5


Diagnóstico de DPOC
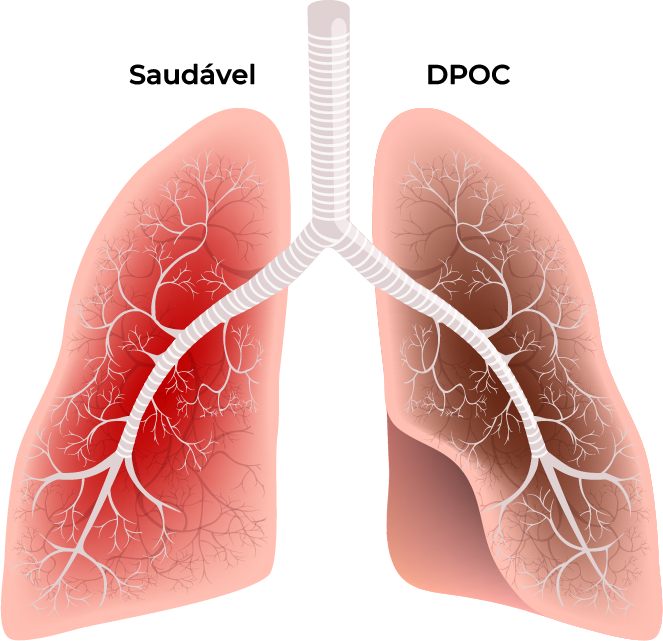
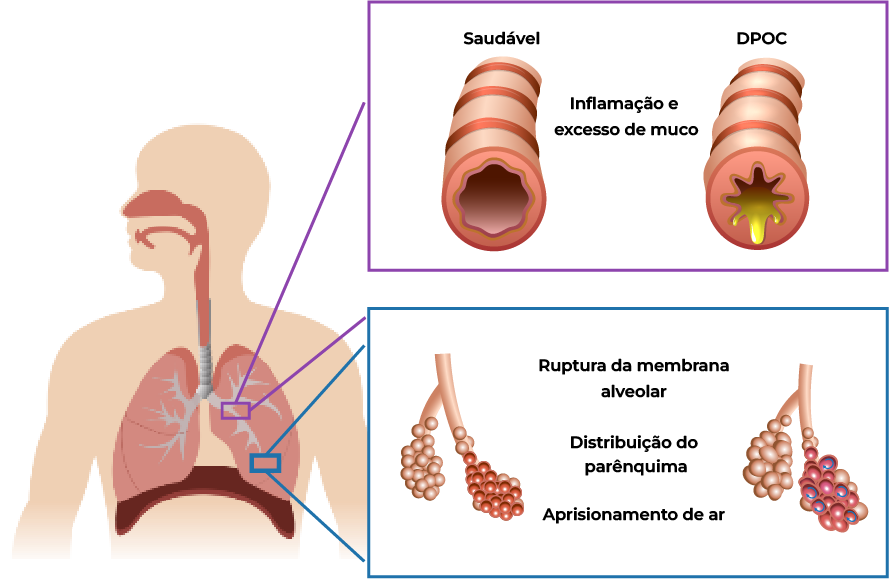
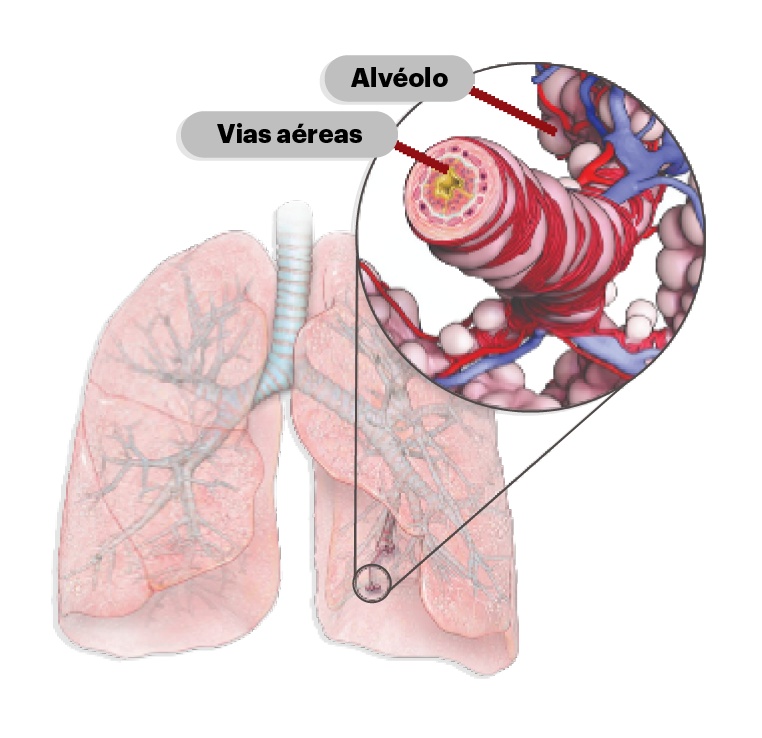
Obstrução aérea na DPOC e caracterização tradicional1
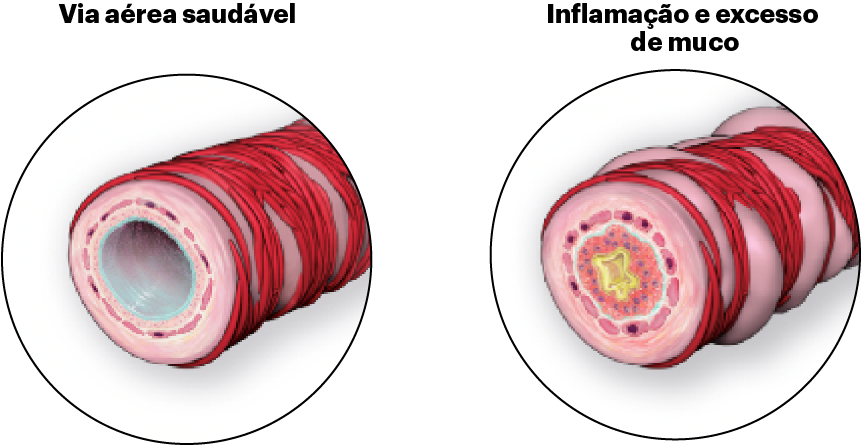
Enfisema
Dano inflamatório crônico às vias aéreas com superprodução e hipersecreção de muco.
● Tosse produtiva crônica
● Muco
● Infecção respiratória
● Exacerbações
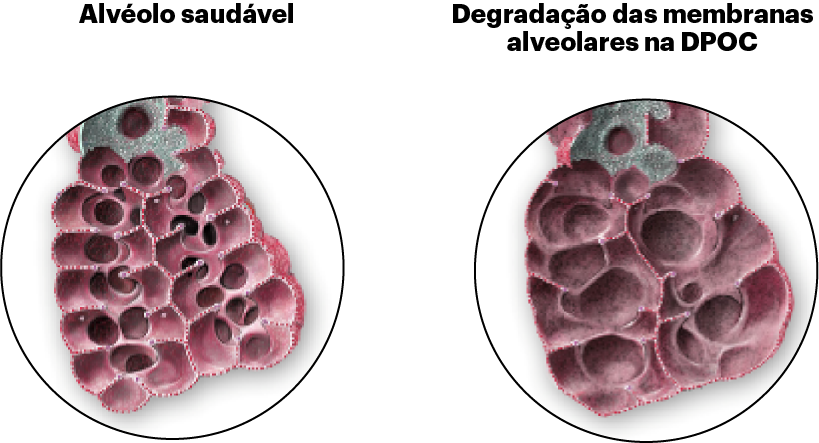
Enfisema
Perda de elasticidade pulmonar, hiperinsuflação pulmonar e destruição dos alvéolos (sacos aéreos) dos pulmões.
● Tosse seca
● Dispneia
● Aprisionamento de ar
● Hiperinsuflação
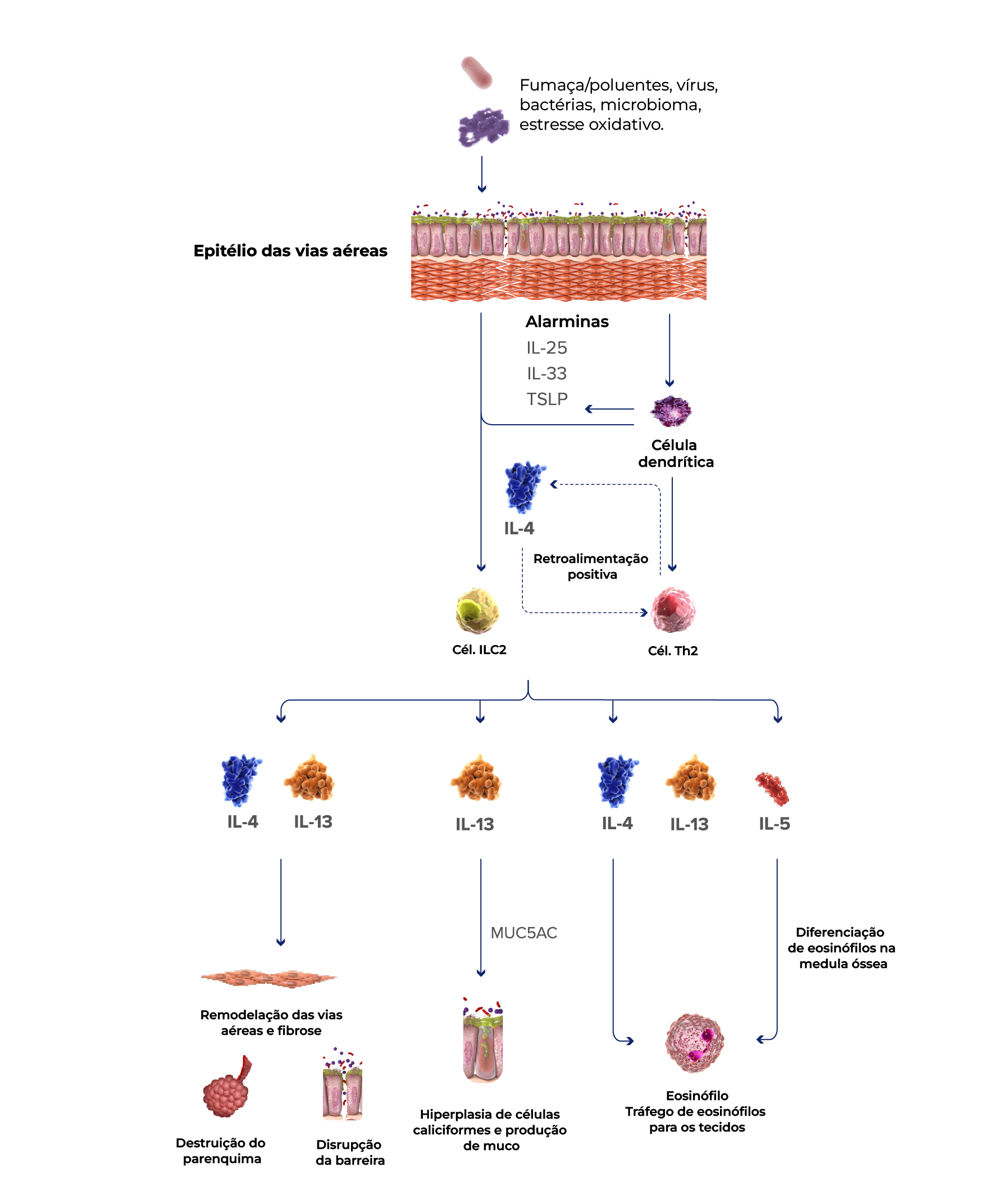
Obstrução do fluxo de ar pode causar:
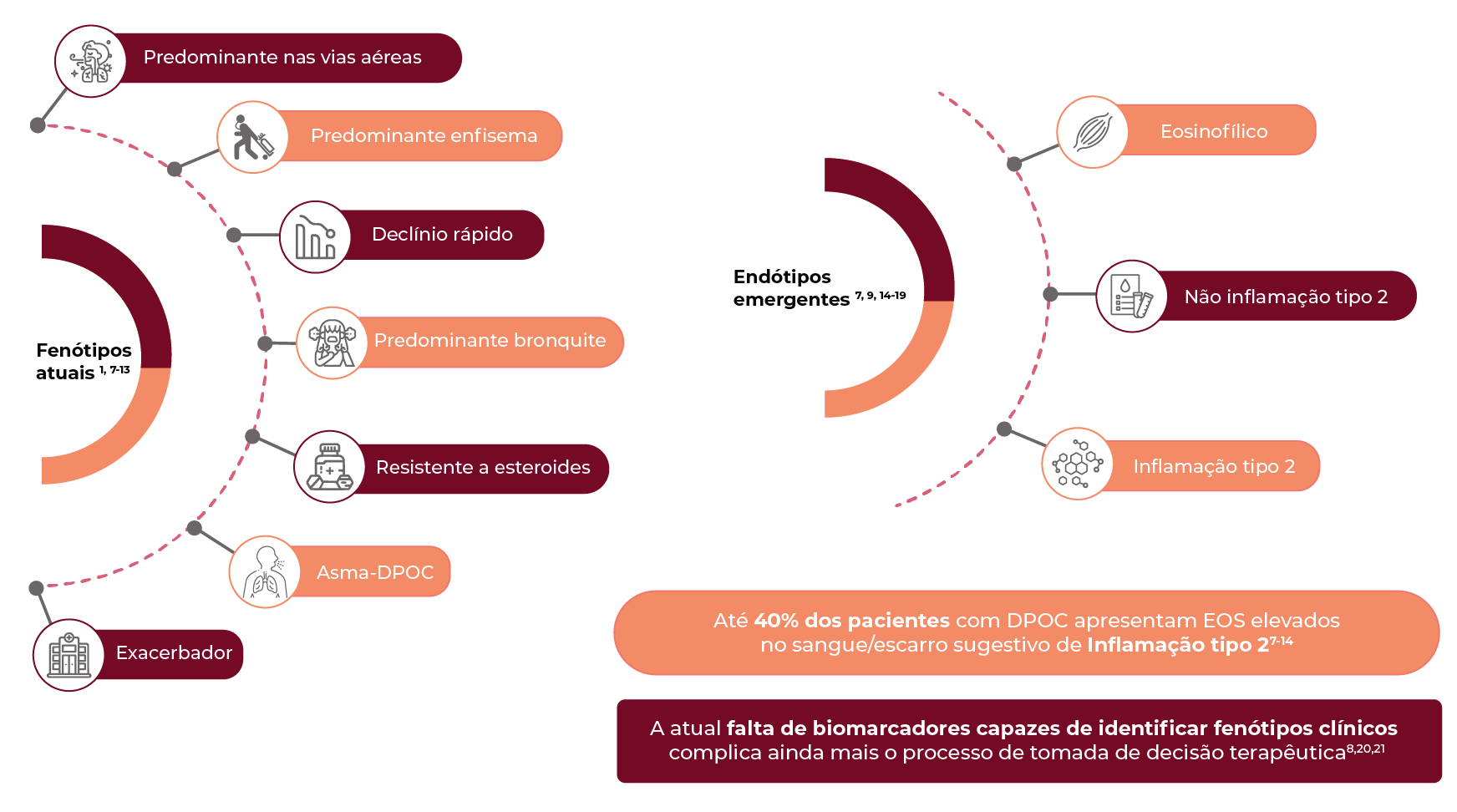
Certas citocinas desempenham papéis importantes na inflamação do tipo 2
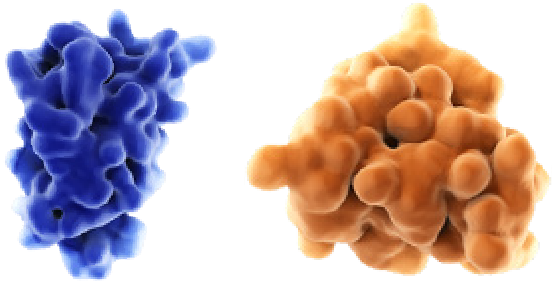
IL-4 e IL-13 impulsionam a atividade das células inflamatórias
IL-4 e IL-13 promovem a ativação e o tráfego de células inflamatórias do tipo 2, incluindo eosinófilos, para os pulmões, o que pode contribuir para o remodelamento das vias respiratórias e a destruição parenquimatosa na DPOC.30,33,35,43-48
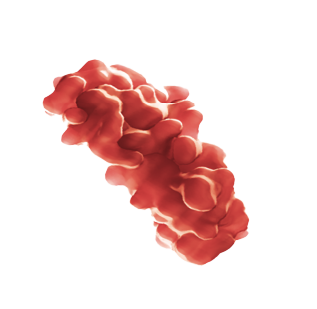
O papel da IL-5
IL-5 é necessário para o crescimento e diferenciação, recrutamento, ativação e sobrevivência de eosinófilos, que podem ser biomarcadores de uma resposta inflamatória do tipo 2 mais ampla.33
- Global Initiative for Chronic Obstructive Lung Disease.
Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2024 report).
Acessado em: 27 de março de 2024. Disponível em: https://goldcopd.org/wp-content/uploads/2024/02/GOLD-2024_v1.2-11Jan24_WMV.pdf. - Sze, M.A., Dimitriu, P.A., Suzuki, M., et al.
Host Response to the Lung Microbiome in Chronic Obstructive Pulmonary Disease.
Am J Respir Crit Care Med. 2015 Aug 15;192(4):438-45. - Lareau, S., Moseson, E., Slatore, C.G.
Exacerbation of COPD.
Am J Respir Crit Care Med. 2018 Dec 1;198(11):P21-P22. - Martinez, F.D.
Early-Life Origins of Chronic Obstructive Pulmonary Disease.
N Engl J Med. 2016 Sep 1;375(9):871-8. - Barnes, P.J.
Inflammatory mechanisms in patients with chronic obstructive pulmonary disease.
J Allergy Clin Immunol. 2016;138(1):16-27. - Linden, D., Guo-Parke, H., Coyle, P.V., et al.
Respiratory viral infection: a potential "missing link" in the pathogenesis of COPD.
Eur Respir Rev. 2019 Mar 14;28(151):180063.7. Bafadhel M, et al. Am J Crit Care Respir Med. 2011;184:662–671. - Bafadhel, M., McKenna, S., Terry, S., et al.
Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers.
Am J Respir Crit Care Med. 2011 Sep 15;184(6):662-71. - Kakavas, S., Kotsiou, O.S., Perlikos, F., et al.
Pulmonary function testing in COPD: looking beyond the curtain of FEV1.
NPJ Prim Care Respir Med. 2021 May 7;31(1):23. - Leigh, R., Pizzichini, M.M., Morris, M.M., et al.
Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment.
Eur Respir J. 2006 May;27(5):964-71. - Alshabanat, A., Zafari, Z., Albanyan, O., et al.
Asthma and COPD Overlap Syndrome (ACOS): A Systematic Review and Meta Analysis.
PLoS One. 2015 Sep 3;10(9):e0136065. - Vestbo, J., Agusti, A., Wouters, E.F., et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints Study Investigators.
Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team.
Am J Respir Crit Care Med. 2014 May 1;189(9):1022-30. - Castaldi, PJ., Dy, J., Ross, J., et al.
Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema.
Thorax. 2014 May;69(5):415-22. - Garcia-Aymerich, J., Gómez, F.P., Benet, M., et al; PAC-COPD Study Group.
Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes.
Thorax. 2011 May;66(5):430-7. - Halpin, D.M.G., de Jong, H.J.I., Carter, V., Skinner D, Price D.
Distribution, Temporal Stability and Appropriateness of Therapy of Patients With COPD in the UK in Relation to GOLD 2019.
EClinicalMedicine. 2019 Jul 24;14:32-41. - Singh, D., Kolsum, U., Brightling, C.E., et al; ECLIPSE investigators.
Eosinophilic inflammation in COPD: prevalence and clinical characteristics.
Eur Respir J. 2014 Dec;44(6):1697-700. - Oshagbemi, O.A., Burden, A.M., Braeken, D.C.W., et al.
Stability of Blood Eosinophils in Patients with Chronic Obstructive Pulmonary Disease and in Control Subjects, and the Impact of Sex, Age, Smoking, and Baseline Counts.
Am J Respir Crit Care Med. 2017 May 15;195(10):1402-1404. - Casanova, C., et al.
Eur Resp J.
2017;50:1701162. - Ajithkumar, C., et al.
Indian J Basic Applied Med Res.
2018;7:223–228. - Bhatt, S.P., Agusti, A., Bafadhel, M., et al.
Phenotypes, Etiotypes, and Endotypes of Exacerbations of Chronic Obstructive Pulmonary Disease.
American Journal of Respiratory and Critical Care Medicine.
2023 Nov 15;208(10):1026-41. - Wu, H.X., Zhuo, K.Q., Cheng, D.Y.
Prevalence and Baseline Clinical Characteristics of Eosinophilic Chronic Obstructive Pulmonary Disease: A Meta-Analysis and Systematic Review.
Front Med (Lausanne). 2019 Dec 10;6:282. - Carolan, B.J., Sutherland, E.R.
Clinical phenotypes of chronic obstructive pulmonary disease and asthma: recent advances.
J Allergy Clin Immunol. 2013 Mar;131(3):627-34; quiz 635. - Oishi, K., Matsunaga, K., Shirai, T., Hirai, K., Gon, Y.
Role of type 2 inflammatory biomarkers in chronic obstructive pulmonary disease.
J Clin Med. 2020;9(8):2670. - Yousuf, A., Ibrahim, W., Greening, N.J., Brightling, C.E.
T2 biologics for chronic obstructive pulmonary disease.
J Allergy Clin Immunol Pract. 2019;7(5):1406-1416. - Barnes, P.J.
Inflammatory endotypes in COPD.
Allergy. 2019;74(7):1249-1256. - Gabryelska, A., Kuna, P., Antczak, A., Białasiewicz, P., Panek, M.
IL-33 mediated inflammation in chronic respiratory diseases—understanding the role of the member of IL-1 superfamily.
Front Immunol. 2019;10:692. - Allinne, J., Scott, G., Lim, W.K., et al.
IL-33 blockade affects mediators of persistence and exacerbation in a model of chronic airway inflammation.
J Allergy Clin Immunol. 2019;144(6):1624-1637.e10. - Calderon, A.A., Dimond, C., Choy, D.F., et al.
Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD.
Eur Respir Rev. 2023;32(167):220144. - Yun, J.H., Lamb, A., Chase, R., et al; for the COPDGene and ECLIPSE Investigators.
Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease.
J Allergy Clin Immunol. 2018;141(6):2037-2047.e10. - Rabe, K.F., Rennard, S., Martinez, F.J., et al.
Targeting type 2 inflammation and epithelial alarmins in chronic obstructive pulmonary disease: a biologics outlook.
American Journal of Respiratory and Critical Care Medicine. 2023 Aug 15;208(4):395-405. - Tajti, G., Gesztelyi, R., Pak, K., et al.
Positive correlation of airway resistance and serum asymmetric dimethylarginine levelin COPD patients with systemic markers of low-grade inflammation.
Int J Chron Obstruct Pulmon Dis. 2017;12:873-88. - Bélanger, M., Couillard, S., Courteau, J., et al.
Eosinophil counts in first COPD hospitalizations: a comparison of health service utilization.
Int J Chron Obstruct Pulmon Dis. 2018;13:3045-3054. - Aghapour, M., Raee, P., Moghaddam, S.J., Hiemstra, P.S., Heijink, I.H.
Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure.
Am J Respir Cell Mol Biol. 2018;58(2):157-169. - Gandhi, N.A., Bennett, B.L., Graham, N.M.H., Pirozzi, G., Stahl, N., Yancopoulos, D.
Targeting proximal drivers of type 2 inflammation in disease.
Nat Rev Drug Discov. 2016;15(1):35-50. - Doyle, A.D., Mukherjee, M., LeSuer, W.E., et al.
Eosinophil-derived IL-13 promotes emphysema.
Eur Respir J. 2019;53(5):1801291. - Cooper, P.R., Poll, C.T., Barnes, P.J., Sturton, R.G.
Involvement of IL-13 in tobacco smoke-induced changes in the structure and function of rate intrapulmonary airways.
Am J Respir Cell Mol Biol. 2010;43(2):220-226. - Arora, S., Dev, K., Agarwal, B., Das, P., Syed, M.A.
Macrophages: their role, activation, and polarization in pulmonary diseases.
Immunobiology. 2018;223(4-5):383-396. - He, S., Xie, L., Lu, J., Sun S.
Characteristics and potential role of M2 macrophages in COPD.
Int J Chron Obstruct Pulmon Dis. 2017;12:3029-3039. - Wang, Z., Bafadhel, M., Haldar, K., et al.
Lung microbiome dynamics in COPD exacerbations.
Eur Respir J. 2016;47(4):1082-1092. - Linden, D., Guo-Parke, H., Coyle, P.V., et al.
Respiratory viral infection: a potential “missing link” in the pathogenesis of COPD.
Eur Respir Rev. 2019;28(151):180063. - Wang, X., Xu, C., Ji, J., et al.
IL-4/IL-13 upregulates Sonic hedgehog expression to induce allergic airway epithelial remodeling.
Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L888-L899. - Saatian, B., Rezaee, F., Desando, S., et al.
Interleukin-4 and interleukin-13 cause barrier dysfunction in human epithelial cells.
Tissue Barriers. 2013;1(2):e24333. - Rosenberg, H.F., Phipps, S., Foster, P.S.
Eosinophil trafficking in allergy and asthma.
J Allergy Clin Immunol. 2007;119(6):1303-1310. - Defrance, T., Carayon, P., Billian, G., et al.
Interleukin 13 is a B cell stimulating factor.
J Exp Med. 1994;179(1):135-143. - Yanagihara, Y., Ikizawa, K., Kajiwara, et al.
Functional significance of IL-4 receptor on B cells in IL-4–induced human IgE production.
J Allergy Clin Immunol. 1995;96(6 pt 2):1145-1151. - Gandhi, N.A., Pirozzi, G., Graham, N.M.H.
Commonality of the IL-4/IL-13 pathway in atopic diseases.
Expert Rev Clin Immunol. 2017;13(5):425-437. - Kaur, D., Hollins, F., Woodman, L., et al.
Mast cells express IL-13Rα1: IL-13 promotes human lung mast cell proliferation and FcεRI expression.
Allergy. 2006;61(9):1047-1053. - Zheng, T., Zhu, Z., Wang, Z., et al.
Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase– and cathepsin-dependent emphysema.
J Clin Invest. 2000;106(9):1081-1093. - Garudadri, S., Woodruff, P.G.
Targeting chronic obstructive pulmonary disease phenotypes, endotypes, and biomarkers.
Ann Am Thorac Soc. 2018;15(suppl 4):S234-S238. - Singanayagam, A., Footitt, J., Marczynski, M., et al.
Airway mucins promote immunopathology in virus-exacerbated chronic obstructive pulmonary disease.
J Clin Invest. 2022;132(8):e12901. - Zhu, Z., Homer, R.J., Wang, Z., et al.
Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production.
J Clin Invest. 1999;103(6):779-788. - Bhatt, S.P., Agusti, A., Bafadhel, M., Christenson, S.A., Bon, J., et al.
Phenotypes, Etiotypes, and Endotypes of Exacerbations of Chronic Obstructive Pulmonary Disease.
American Journal of Respiratory and Critical Care Medicine. 2023 Nov 15;208(10):1026-41. - Brightling, C.E., Saha, S., Hollins, F.
Interleukin-13: prospects for new treatment.
Clin Exp Allergy. 2010;40(1):42-49.
Referências:
 nas abas abaixo. A aba em destaque mostrará o conteúdo correspondente a cada título:
nas abas abaixo. A aba em destaque mostrará o conteúdo correspondente a cada título: